| We aim to advance the understanding of combustion physics and chemistry of green fuels at low temperature, high pressure, and high speed for advanced engines and propulsion, and to develop novel non-equilibrium plasma aided combustion and electrified manufacturing technologies to decarbonize energy conversion and industrial manufacturing. |
| Cool, Warm, and Hot Flames God has created 3 different kinds of flames. Until recently, we are able to observe stabilized cool flames and warm flames in the laboratory by using ozone and plasma activation. [paper] |  |
| Combustion at extreme pressure (100 atm): E-fuels and biofuels E-fuel such as methanol and H2 has attracted considerable attention with zero greenhouse gas emissions. A new supercritical pressure jet-stirred reactor (SP-JSR) was developed and measured methanol and methane oxidation at pressures up to 100 atm. The experimental results show a negative temperature coefficient (NTC) behavior for both methanol and methane. [Paper] | 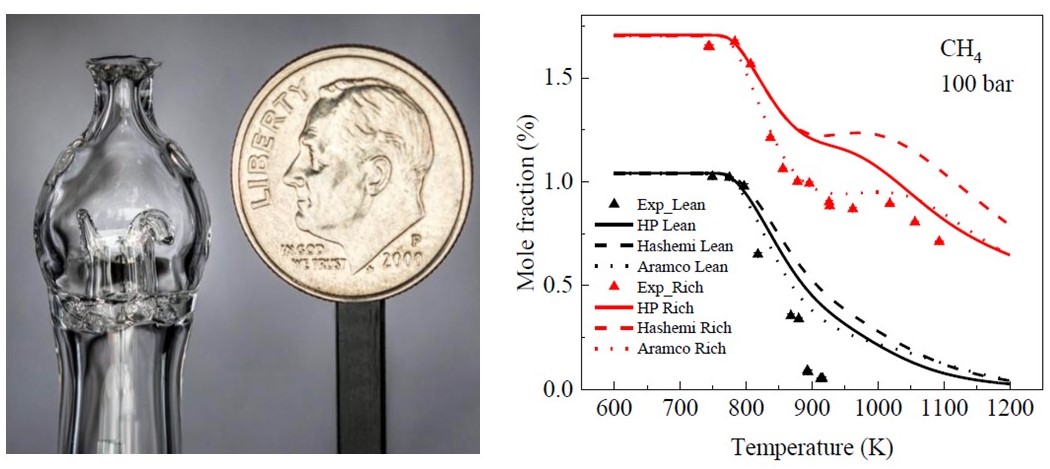 |
| Faraday Rotational Spectroscopy and HO2 reaction kinetics Key HO2 radical-radical reactions for intermediate temperature combustion chemistry were measured in situ for the first time in a photolysis Herriott cell by using mid-IR Faraday rotation spectroscopy (FRS) and UV-IR direct absorption spectroscopy (DAS). The microsecond time-resolved diagnostic technique enabled the direct reaction rate measurements and reduced the uncertainty of HO2 chemistry. [Paper] | 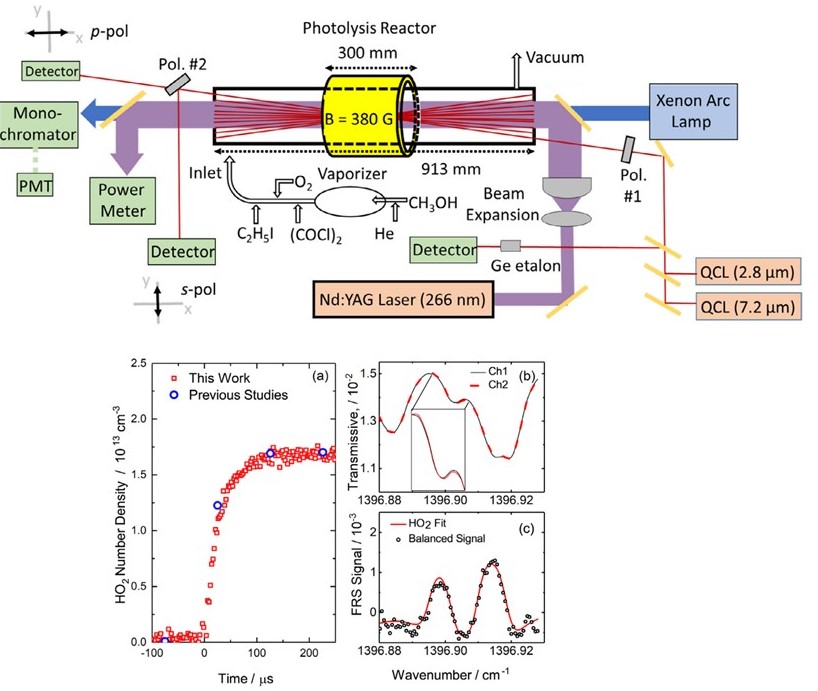 |
| Plasma assisted detonation transition Plasma can generate active species such as excited molecules and radicals to accelerate combustion and detonation. The figure in the right shows that plasma discharge can dramatically enhance deflagration to detonation transition. [paper] |  |
| Plasma-assisted low-temperature NH3 oxidation and NOx formation Ammonia represents one of most promising zero-carbon energy solutions to address the increasingly urgent climate change problem. However, the existing utilization of ammonia faces significant challenges from low chemical reactivity and high NOx/N2O emissions. In this work, for the first time, we explored plasma assisted ammonia oxidation at room temperature with a focus on unveiling non-equilibrium NOx/N2O reaction pathways by combining in-situ laser diagnostics with plasma modeling. We found that the nonequilibrium plasma controls the NOx formation by supplying O/H/N atoms via electron-impact dissociation and collisional quenching of excited species. [Paper][Paper] | 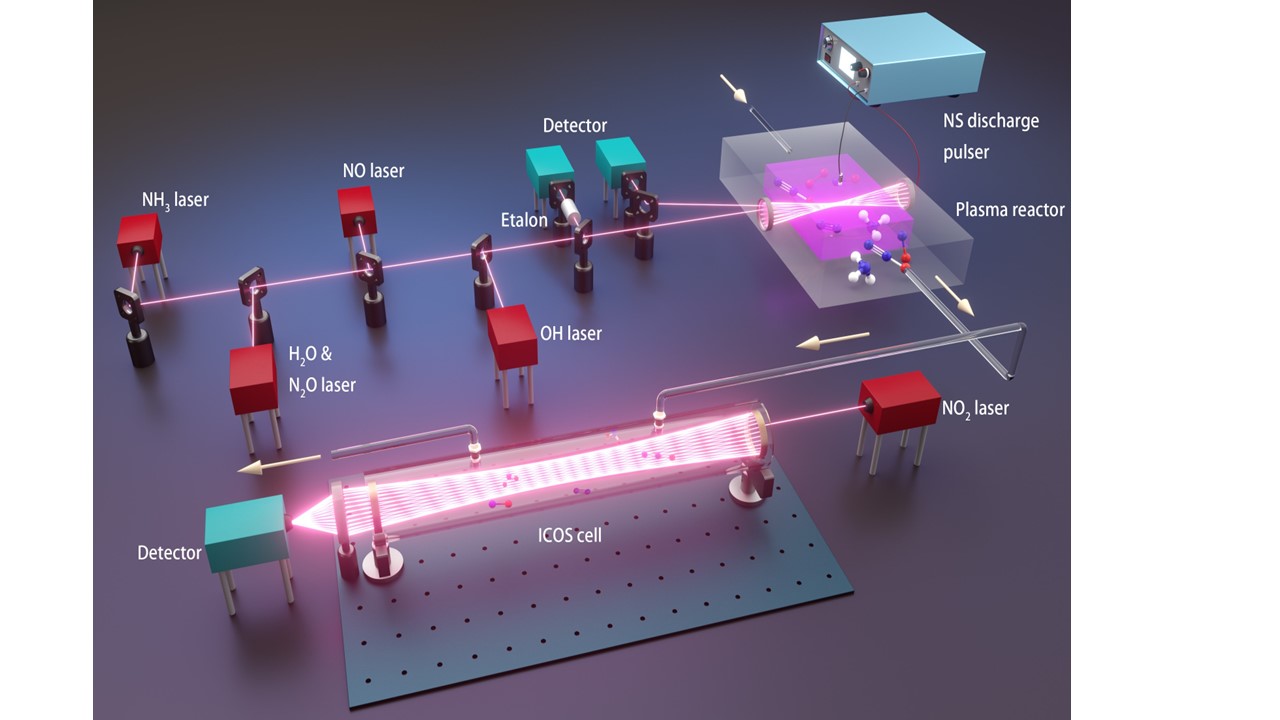 |
| Enhancements of electric field and afterglow using ferroelectric plasma Manipulating surface charge, electric field, and plasma afterglow in a nonequilibrium plasma is critical to control plasma-surface interaction for plasma catalysis and manufacturing. Here, we show enhancements of surface charge, electric field during breakdown, and afterglow by ferroelectric barrier discharge. The results show that the ferroelectrics manifest spontaneous electric polarization to increase the surface charge by two orders of magnitude compared to discharge with an alumina barrier. Moreover, due to the existence of surface charge, the ferroelectric electrode extends the afterglow time and makes discharge sustained longer. The present results show that ferroelectric barrier discharge offers a promising technique to tune plasma properties for efficient plasma catalysis and electrified manufacturing. [Paper] | 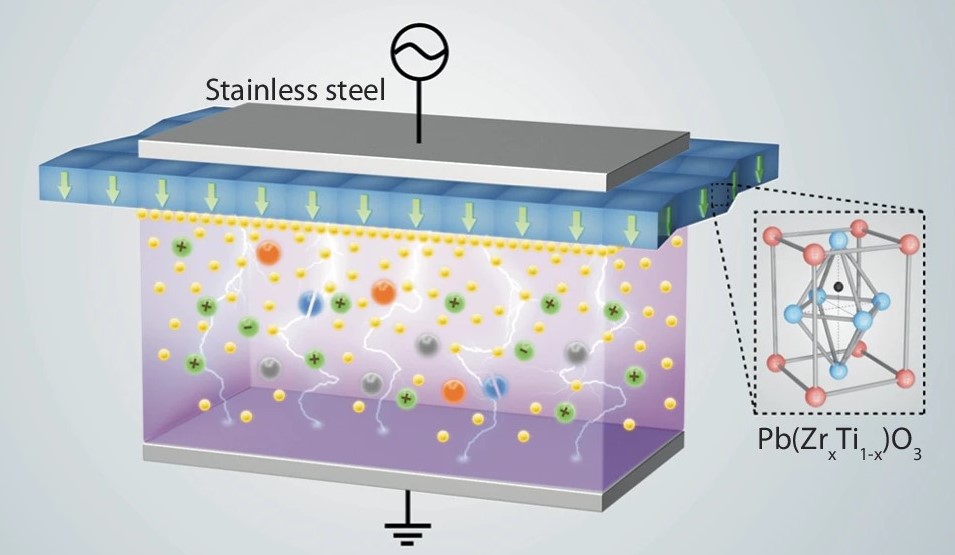 |
| Plasma aided extreme materials manufacturing We invented a novel carbon-fibre-tip-enhanced plasma that enable the generation of a uniform, ultra-high temperature and stable plasma (up to 8,000 K) at atmospheric pressure. We have successfully synthesized various extreme materials in seconds, including ultra-high-temperature ceramics (for example, hafnium carbonitride) and refractory metal alloys. [Paper] |  |
| Catalyst-free and Far-from-equilibrium Depolymerization of Plastics via Electrified Spatiotemporal Heating We developed a catalyst-free, far-from-equilibrium depolymerization method via electrified spatiotemporal heating that enables us to generate monomers from commodity plastics with high yields. Using this approach, we depolymerized polypropylene and polyethylene terephthalate to their monomers with yields of ~36% and ~43%, respectively, which are among the highest compared to conventional thermochemical methods, even those that use catalysts (e.g., ~10–20%). Additionally, the process features good scalability and reduced CO2 emissions compared to conventional methods. Overall, this novel electrified spatiotemporal heating approach offers a promising and practical solution to the global plastic waste problem. [Paper] | 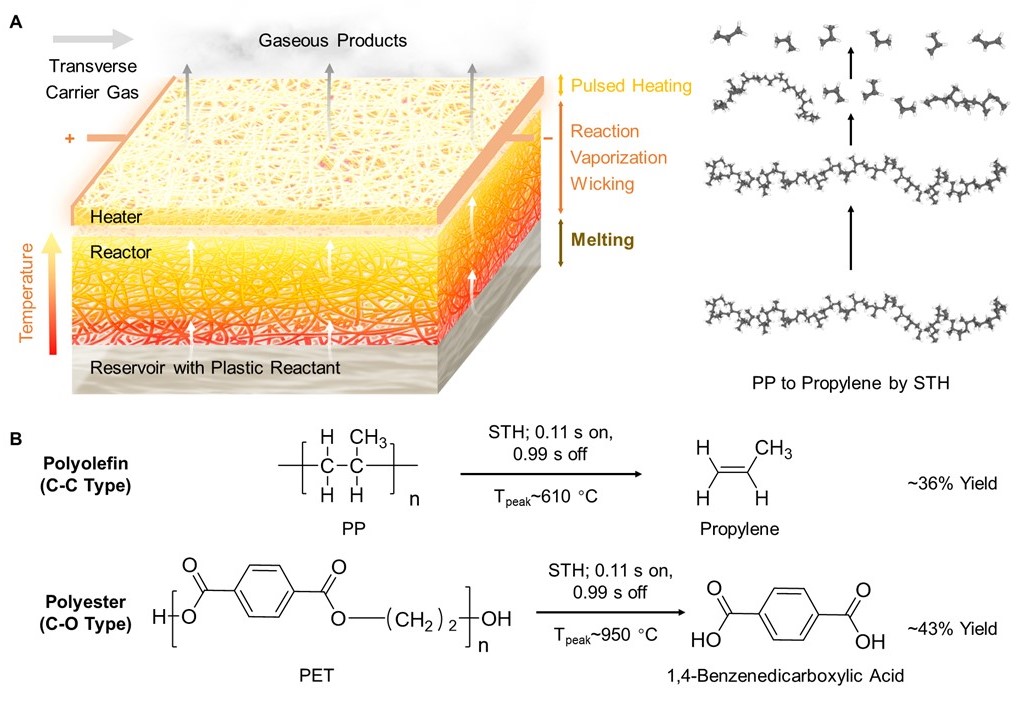 |
| Plasma-Assisted Catalysis for NH3 Synthesis: NNH pathway Ammonia synthesis at atmospheric pressure was investigated in a coaxial dielectric barrier discharge (DBD) plasma reactor with porous γ-Al2O3, 5 wt % Ru/γ-Al2O3, or 5 wt % Co/γ-Al2O3 catalyst particles. Gas-phase species NNH and N2H2 were first identified in plasma-assisted ammonia synthesis. These observations point to the importance of NNH and N2H2 in plasma-assisted surface reactions in ammonia synthesis. Reaction pathways of direct adsorption of gas-phase NNH and N2H2 on solid surfaces and subsequent reactions were proposed. [Paper] |  |
| Plasma thermal chemical instability When a plasma discharge occurs in a reactive mixture, the plasma interaction with combustion chemistry will dramatically change the plasma development and results in “plasma thermal chemical instability. We have developed the theory as well as experimental and computational validation of the existence of this instability. The figure on the right shows the comparison of the plasma instability in air (left) and dimethyl ether (right). [Paper] | 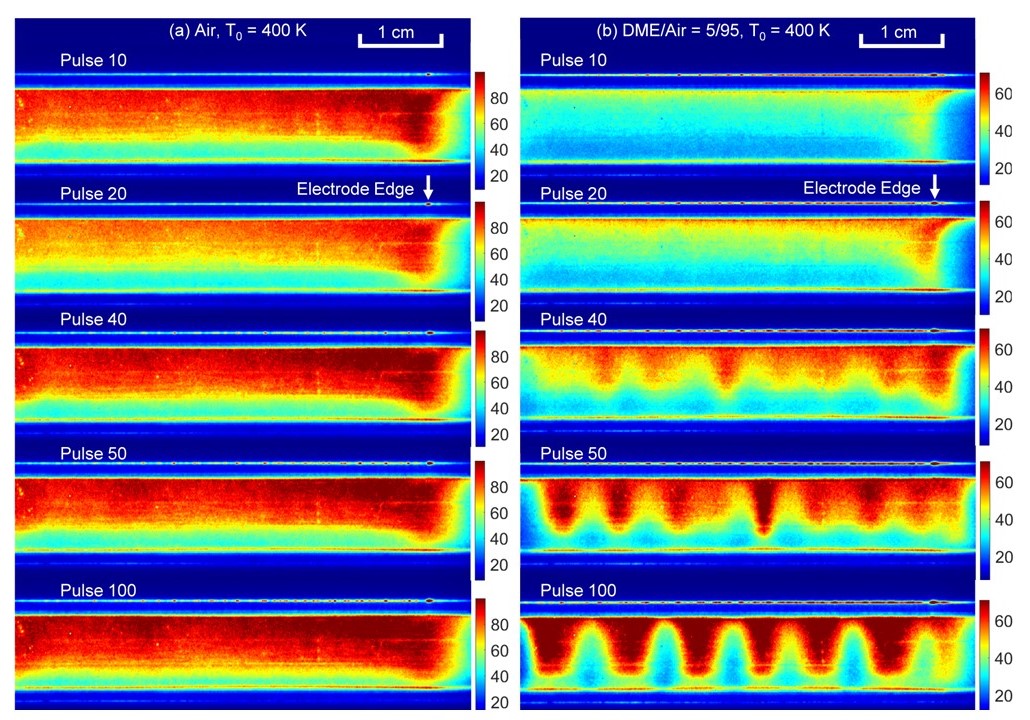 |
| Rotational fs/ps CARS coherence beating for non-equilibrium temperature measurement We report the development of a simple and sensitive two-beam hybrid femtosecond/picosecond pure rotational coherent anti-Stokes Raman scattering (fs/ps CARS) method to simultaneously measure the rotational and vibrational temperatures of diatomic molecules. Rotation–vibration non-equilibrium plays a key role in the chemistry and thermalization in low-temperature plasmas as well as thermal loading of hypersonic vehicles. [Paper] |  |
| Sensitive and single-shot OH and temperature measurements by femtosecond cavity enhanced absorption spectroscopy We used cavity enhanced absorption spectroscopy (CEAS) and femtosecond (fs) pulsed laser to enable sensitive single-shot measurements of OH concentration and temperature with a time resolution of ~180 nanoseconds (ns) in LTPs. Such combination leveraged several diagnostic benefits. With the appropriately designed cavity, an absorption gain of ~66 was achieved, enhancing the actual OH detection limit by ~55× to the 1011 cm-3 level (sub-ppm in this work) compared to single-pass absorption. [Paper] | 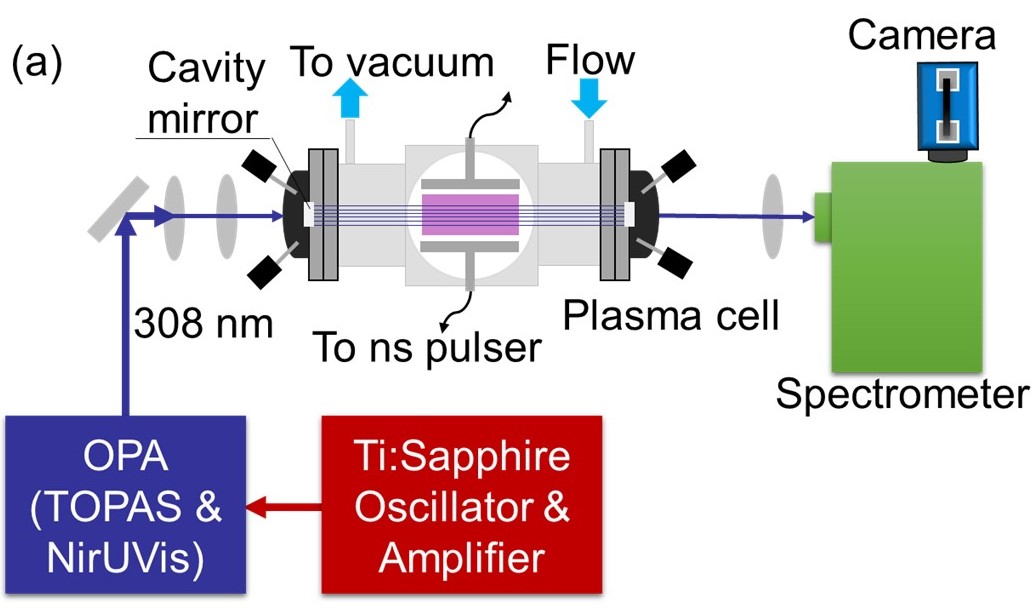 |
| 1.1 The flammability limit and near or sub-limit combustion Advanced engines and propulsion systems often work at extreme conditions near or below the flammability limit to improved energy efficiency and reduce emission. The flammability limit is governed by fuel chemistry and flame radiation. Due to the preferential diffusion effect, fuels with smaller Lewis numbers can burn below the flammability limit if stretched. The general flammability limit diagram is given by the G-curve (see the figure on the right for CH4/air). E is the the fundamental flammability limit of methane, B is the sublimit of stretched methane flame. That is, a streched flame can burn leaner than unstretched flames. This was observed in microgravity counterflow flame experiment. | 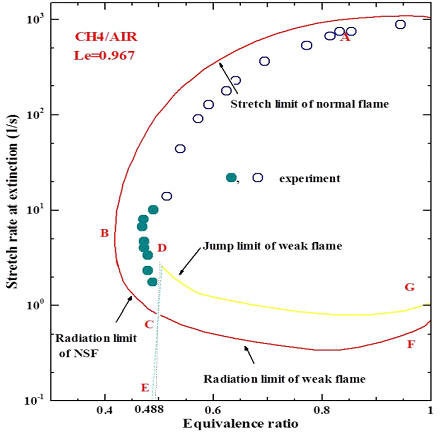 |
| 1.2 The minimum ignition energy and critical flame radius Ignition and ignition to flame transition in engines are governed by the so called minimum ignition energy (MIE). Despite research in several decades, the physics behind MIE was not understood. Recently, by introducing radiation and flame stretch, we discovered that MIE is governed by the critical flame initiation radius, which is a function of pressure, Lewis number, and fuel activation energy (see Figure on the right). These criterions are experimentally measured and provide guidance for engine design. This critical flame initiation radius was measured for liquid fuels. |  The critical flame radius vs. Lewis number |
| 1.3 Alternative fuels, Surrogate fuels, Radical Index Energy sustainability and climate change present a urgent call for renewable fuels. These new fuels such as synfuels as well as solar fuels and biomass derived alcohol and biodiesel have hundreds of different molecular structures and distinctive combustion and emission characteristics. It is important to understand the combustion characteristics of fuels with different molecular structures and develop a surrogate fuel model with a few selected components to mimic the real fuel performance. Our research focus on the measurements of flame properties (e.g. flame speeds, ignition and extinction limit, flame structure, and speciation) by using counterflow flames, high pressure spherical bomb, and jet stirred reactor; the development of generic correlation between flame extinction and molecular structure by introducing Radical Index; Transport weighted enthalpy (TWE), and the development of generic surrogate fuel models to mimic real fuels, and validation of kinetic mechanism of jet fuel, methyl esters (biodiesel), and alcohols. |  Correlation between extinction limit and radical index (Ri) and transport weighted enthalpy (TWE) for different fuels |
| 1.4 High pressure flame chemistry Combustion in engines is governed by ignition, extinction, and flame propagation. At high pressures, the reaction pathways change significantly. For example, the HO2 related kinetic pathways led to negative pressure dependence of flame speed and H2O2 decomposition becomes dominant. The lab has been developing high pressure spherical flames, counterflow flames, and high pressure jet stirred reactors to study the high pressure flame chemistry for H2 and high pressure mechanism (HP-Mech). | 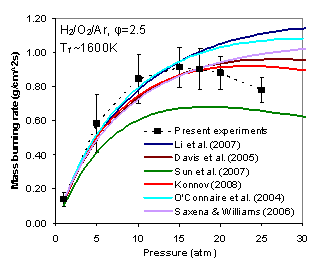 Measured and predicted hydrogen burning rates |
| 1.5 Cool Flames and Low Temperature Fuel Oxidation Cool flame has been regarded later as a key process for engine knock, motivating extensive studies on large hydrocarbon low temperature chemistries. However, establishing a stable cool flame is extremely challenging. A novel method to establish self-sustaining cool diffusion flames with well-defined boundary conditions has been experimentally demonstrated by using ozone and plasma. For more, pls read, [1] Won, et al., Self-Sustaining n-Heptane Cool Diffusion Flames Activated by Ozone, Proceedings of Combustion Institute, 35, Accepted, 2014 [2]Sun, W. et al. http://dx.doi.org/10.1016/j.combustflame.2014.01.028 | 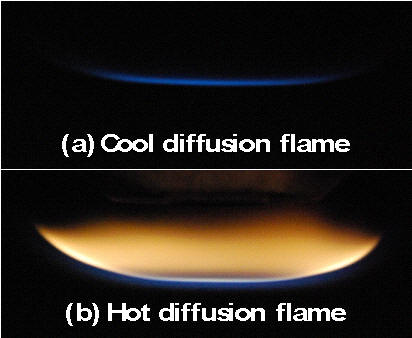 Comparison of a hot and a cool diffusion n-heptane flame |
| 1.6 Turbulence Combustion with Low Temperature Chemistry Combustion in practical gas turbine engines, after-burners, and internal combustion engines are governed by ignition, and turbulent premixed and partially premixed flames at elevated temperature and pressure at high Reynolds numbers. However, little is known about the role of low temperature fuel chemistry on turbulent combustion and propagation speeds. The results suggest that contrary to the previous studies, the turbulent flame regimes and burning velocities for fuels with low temperature chemistry can have multiple values and may not be uniquely defined at elevated temperatures. A new high temperature, high Reynolds number, Reactor Assisted Turbulent Slot (RATS) burner has been developed to investigate turbulent flame regimes and burning rates for large hydrocarbon transportation fuels, which exhibit strong low temperature chemistry behavior. It is found for the first time that for n-heptane/air mixtures there are four unique turbulent flame regimes. Moreover, low temperature ignition can induce flame flashback. |   |
| 1.7 Combustion diagnostics (PIV, PLIF, Rayleigh, IR absorption, FRS, MBMS) Combustion diagnostics is critical to provide species information to valid chemical kinetic mechanism. We have been developing single photon and two-photon laser induced fluorescence, Rayleigh scattering, UV and mid-infrared laser absorption spectroscopy, and molecular beam mass spectrometry to detect radicals, intermediate species, reactants, and products in flames and reactors. |  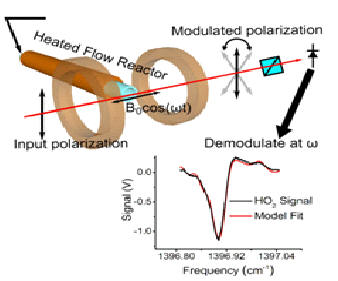 HO2 diagnostics using mid-IR Faraday Rotational Spectroscopy (FRS) |
| 2. Non-Equilibrium Plasma Assisted Combustion The development of scramjet engines for supersonic propulsion requires ignition and complete combustion to be achieved within one millisecond. Non-equilibrium plasma provides a new opportunity to enhance ignition. However, despite of many technological developments and claims in the literature, the kinetic mechanism of plasma based combustion enhancement was not well understood. Our research has been focused on the understanding of kinetic and transport role of electronically excited species (singlet delta, long lifetime catalytic species, and active radicals generated by gliding arc, microwave, and nanosecond discharge on ignition and flame extinction. |  |
| 3. Microscale Energy Conversion Microscale energy conversion has a broad application on micro-power generation, surveillance propulsion, hydrogen synthesis, chemical sensors, and solid oxide fuel cells. On such a small scale, the surface to volume ratio significantly increases and the thermal inertia time of the reactor structure becomes comparable to the thermal diffusion time of the reactants. As such, increased heat loss, flame-structure coupling and radical quenching on the surfaces lead to rich and new flame dynamics. We have been developing a number of theoretical models and conducted experiments to describe the impact of heat recirculation and radical quenching on mesoscale combustion. The theory predicted new flame regimes such as weak flame, normal flame, as well as flame streets. Read more |  The methane-air diffusion flame street formed in a mesoscale channel |
| 4. Functional Nano-materials The sharp and up-converting properties of rare earth doped nanophosphors (e.g. NaYF4:Ln3+) provide new opportunities for temperature imaging, bioimaging, counterfeit security, solar cells and LEDs. However, optical quenching and monodisperse synthesis are the great challenges. We developed new flame based and high temperature in-solution based down-conversion and up-conversion nanophosphor synthesis methods to obtain monodispersed nanocrystals from 5 nm to 250 nm. We also developed a kinetic model to control the particle size monotonically by using a single parameter of lanthanide ion to Na ratio. By conducting dynamic luminescence time decay measurements, nonlinear luminescence power dependence analysis, and Raman scattering at different crystal sizes, we proposed that it is the surface quenching but the quantum confinement responsible for the luminescence decrease with the decrease of particle size. By using the temperature dependency of luminescence, we developed a simultaneous particle velocimetry and thermo(PIVT) method to measure temperature and velocity in reactive flows at the same time. By collaborating with Wole Soboyejo in MAE, Robert Prud’homme in Chemical Engineering, and Robert Austin in Physics, we successfully demonstrated the singlet oxygen production of biofunctionalized nanoparticles and cancer killing ability using near infrared photodynamic therapy. [Click here for more details] | 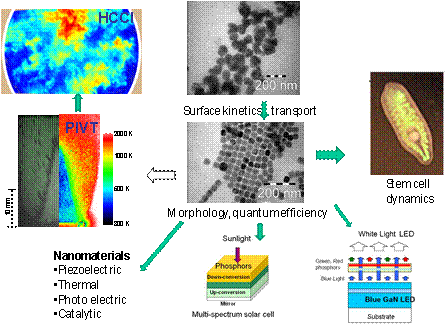 |

Research News